"Sakura-san, in the old days, all CRFs were made of paper and bound like notebooks, so when the clinical monitor made an appointment with the investigator, he/she would take the stack of CRFs to the clinical site..."
The president's old story has started again. Come on, this is the third time I've heard that story, so I know it well. And at the end, when he asked the investigator for his signature stamp, he said, "Why do I have to use my signature stamp? I won't take any responsibility."
"At the end, when I asked the investigator at a certain T Hospital for his signature stamp, he asked me why I have to use my signature stamp, and there was a fuss. That doctor was the primary investigator, but..."
"Then, EDC came out, and before you know it, the paper CRFs were gone."
Oh, there's more to come today. Usually, I somehow managed to convince the doctor at a certain T Hospital to get a signature stamp, but it was difficult in the days of paper CRFs. The president's story usually ended with this episode, but it took an unexpected turn, so I continued to listen to it for a while longer.
"Well, it seems that EDC is coming to an end and will be replaced by SDC, so it's amazing how much technology advances. In fact, I don't think things would have moved so quickly if there hadn't been a Corona pandemic five years ago."
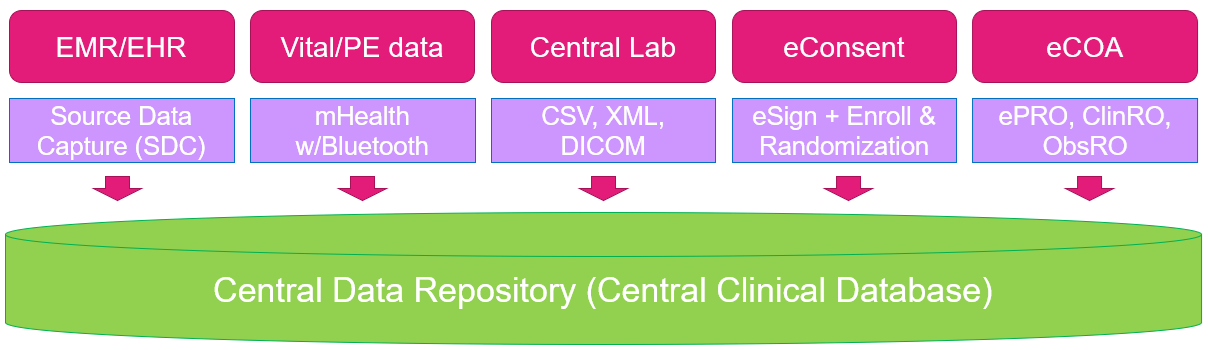
The Corona crisis happened when I was a second-year university student, and since then everything in the world has been changed. By the way, SDC stands for Source Data Capture, and it is a system that electronically connects systems such as electronic medical record data and clinical trial database, so that the medical record data can be directly absorbed into the clinical trial database.
"The world has been changed all at once. It was a struggle to go to the hospital back then."
"But perhaps reflecting this situation, the movement towards digital transformation has progressed even further, and it was around that time that most data could be collected electronically. Until recently, clinical sites wrote and entered data for clinical development on paper CRFs and EDCs, and collected the transcribed data, but only recently SDC has become widespread, and now most systems are connected electronically and data flows automatically. Thanks to this, I think the burden on medical facilities has been greatly reduced, the quality of the data itself has improved, and there is no longer a need to visit facilities multiple times for SDV, so it's all good."
"It looks like data scientists like us will be busier in the future."
As I said this, I remembered a clinical trial I participated in a long time ago. At that time, Rock was there and collected my data every day. The data collected by Rock was directly sent to a central database and stored there, and because the data itself was the source data, there was no need to do SDV. In that sense, it was amazing that clinical trial was conducted using the robots at that time.