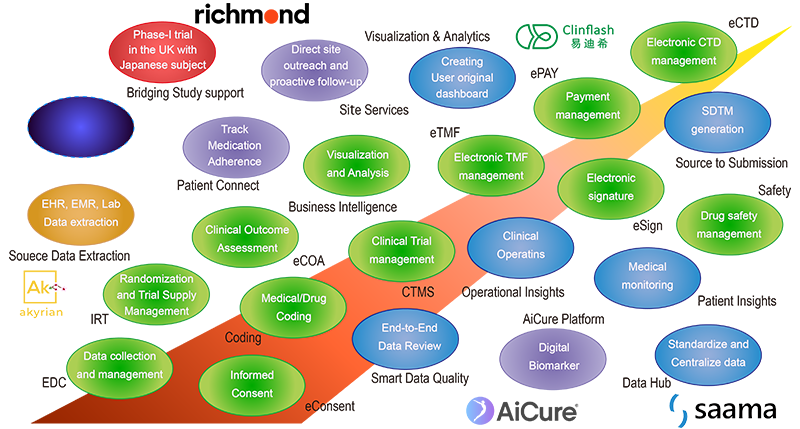
As shown below, Magoneko has a wide range of products covering everything from the early stages of Clinical Development to New Drug application. Our solution lineup includes basic solutions such as EDC and CTMS that are already used as standard in Clinical Development work, as well as cutting-edge solutions that have been developed to solve conventional problems and are expected to become mainstream in the future. We are offering these two types of solutions.
The core solution is Clinflash® Cloud, which covers almost all operations necessary for Clinical Development. We also offer products from AiCure, Akyrian Systems, InHandPlus, Kenosha AI, Saama and Sequentia Biotech as solutions for business reforms and efficiency improvements using cutting-edge AI-based technology. We have also begun offering Extra Horizon's solutions as a platform for the development and deployment of SaMD, which has been attracting attention recently. In addition to these IT solutions, through our business partnership with London-based Richmond Pharmacology, we are now able to assist with conducting Phase I clinical trials including Japanese subjects and gene therapy Japanese patients. This will enable us to further speed up and streamline clinical development in Japan, helping to resolve issues such as drug lag and drug loss.
In addition, the product lineup is being expanded gradually, and we will notify you of any products that are scheduled to be added (products surrounded by dashed lines in the chart below) as appropriate through our web page.
During COVID-19, with a growing demand for remote and decentralized solutions, AiCure was seen as a unique value because of its years of experience deploying and supporting patient-facing software. AiCure responded by developing compliant telemedicine solutions for its sponsors. Also, AiCure launched Clinical Site Services (CSS) to relieve technology burden at sites.
Today, AiCure continues to innovate and broaden its market presence as a platform for all things patient-facing. This includes eConsent, ePRO/eCOA, telemedicine, AI-driven patient engagement via two-way text, and of course, medication adherence and digital biomarkers.
AiCure has a proven record with dozens of clinical trials sponsored by the most prominent Big Pharma companies in the world. Its AI-platform has been deployed in over 40 languages. Importantly, AiCure has supported studies in all phases of drug development leading to FDA submissions, projects in post-marketing research, city- and state-wide deployments in public health, and large US Government and military contracts.
They created Akyrian Systems, and the result of years in R&D led to Akyrian’s flagship product, Source Data Extraction or SDE, a patented clinical data platform that is now poised to significantly reduce clinical trial duration and overall cost.
Services for Japanese customers are provided by the system within a cloud data center in Japan.
| Category/Product Name | Function |
|---|---|
| Cloud Data Solutions | |
| Clinflash® EDC | Data collection and management of Clinical trial data |
| Clinflash® IRT | Randomization and Trial Supply Management |
| Clinflash® Coder | Medical and Drug Coding |
| Clinflash® BI | Data visualization, analysis and central monitoring |
| Digital Cloud Solutions | |
| Clinflash® eCOA | Electronic Clinical Outcome Assessment |
| Clinflash® eCTD | Electronic Common Technical Document |
| Clinflash® ePAY | Clinical Trial Electronic Payment |
| Clinflash® eSign | Clinical Trial Electronic Signature |
| Clinflash® eConsent | Electronic Informed Consent |
| Project Management Cloud Solution | |
| Clinflash® CTMS | Clinical Trial Management System |
| Clinflash® eTMF | Electronic Trial Master File |
| Pharmacovigilance Cloud Solution | |
| Clinflash® Safety | Pharmacovigilance Safety Database |
By breaking the recurring barriers involved in building a MedTech solution, we help get digital health products to the people who need them as quickly as possible, improving the quality of healthcare and making a lasting impact.
Specific examples include tracking medication, detecting scratching and tremors, recording each meal and calculating calories, and analyzing the effects of food. It is expected to be applied to clinical trials and research, as well as dietary advice and DCT.
This product has several features, including its low price (please inquire about the price), fast data updates (you can search for notifications from the previous day), and the display of the type of search being performed based on the user's prompt. In addition, since the LLM is tuned specifically for regulatory information, the hallucination is minimized and you can quickly access the desired information.
Richmond Pharmacology has over 20 years of experience conducting over 100 Phase-I trials in London targeting Japanese volunteers, and is a very unique CRO with the largest Japanese database (24,000 people) of any facility based in Europe, and has a track record of being used by many clients, particularly those planning international joint clinical trials. In addition, recently, trials targeting specific patient cohorts have accounted for approximately 60% of the total number of trials, and the company is also focusing on clinical trials in the fields of gene silencing and gene editing. While accurately grasping the needs of the times, the company is working hard to accelerate research using adaptive designs and provide "Faster Answers."
Saama uses artificial intelligence (AI), along with advanced analytics, to automate key clinical development processes and surface actionable insights. This allows customers to accelerate their time to market and eliminate manual, resource-intensive processes.
Saama accomplishes this mission through the combination of our strong portfolio of SaaS-based solutions, ready for deployment out-of-the box, as well as our ability to work with your organization to build and deploy custom AI and advanced analytics-based solutions. This combination of capabilities means we can cover the entire spectrum of clinical development and commercialization.
| Category/Product Name | Function |
|---|---|
| Data centralization and standardization platform | |
| Data Hub | Data integration platform for all applications below |
| AI assisted applications for Clinical development processes | |
| Smart Data Quality | AI assisted End-to-End data review |
| Operational Insights | AI assisted Operational review |
| Patient Insights | AI assisted patient journey review |
| Source to Submission | AI assisted SDTM transformation |
| BRAIN | Integrated program and dataset management environment |
| Document Generation platform | |
| Protocol Authoring | Protocol generation and management |
| CSR Authoring | CSR generation and management |
For the pharmaceutical industry, applications include designing personalized medicine and discovering new biomarkers using Omics data and clinical data (RWD and clinical trial data), but we believe that it can also be applied to agriculture, livestock farming, environmental science, and more.