In clinical trials, costs of clinical monitoring generally account for 30% to 40% of the total cost. Some older literature states that it is 50%, but recently this percentage seems to be decreasing little by little due to the spread of risk-based approaches and the reduction in the number of site visits due to the COVID-19 pandemic. However, it remains a significant cost factor that cannot be ignored.
Clinical monitoring is an important activity to confirm whether the clinical trial is being conducted in compliance with regulations and study protocol, whether subjects are being enrolled as planned, and ensuring the quality of trial data and the safety of subjects. In particular, it can be said that it takes a considerable amount of time and money as a cost related to SDV (Source Data Verification) to check and verify whether data is accurately transferred from Medical Records to EDC.
On the other hand, data verification work is a quite simple task, not a highly scientific activity. It can be said that it is a considerable waste to implement this at a huge cost, including valuable time of talented people and travel expenses.
The Source Data Extraction (SDE) from Akyrian Systems (Akyrian) is an innovative solution that increases cost efficiency in Clinical Trials. The SDE improves data quality, shortens clinical trial execution time, and reduces costs by completely eliminating SDV.
Similar solutions have existed in the past, such as HL7 adaptor for EDC or Source Data Capture. In addition, data linkage systems for electronic medical records and EDCs using FHIR presented by HL7 are also existing. However, although data integration with Electronic Medical Records has been considered for over 20 years, but there are currently only a limited number of successful cases.
Some information sources say that electronic medical record linkage using FHIR is becoming popular in the United States, but when searching on Google, there seem to be few successful examples. Additionally, this is a case study from Japan, according to the report of DS Subcommittee TF 1-2 of the JPMA (published in June 2023), the survey of 50 companies revealed that only one company had implemented data linkage between electronic medical records and EDC.
Now, let's get back to Akyrian's SDE. The biggest feature of this system is that when linking electronic medical record data to EDC, it uses a paradoxical data linkage method in which electronic data is not linked between systems. Specifically, we use technology that imports data from electronic medical records as image data, and at that time converts character strings made up of bitmaps on the image data into data using OCR and AI. By using this method, it is possible to import not only data in electronic medical records, but also data written on paper or PDF. For example, it can be used in cases where sites still use paper medical records, or where Lab test results taken in-hospital are output on paper as test slips.
Furthermore, there is a function to store the target medical records and test slips as image data at the time of data capture, and use them as part of the audit trail. It also has a function that allows you to check both data on the screen.
These features completely eliminate the task of transcribing data needed for clinical trials from medical records. Additionally, regarding concerns about personal information being included in image data, there is a function that uses AI to black out all image data when it is imported into the system, eliminating the risk of personal information being visible.
Now, let's take a look at the results of Akyrian's calculations of how effective it would be if SDV were completely eliminated (figures below)
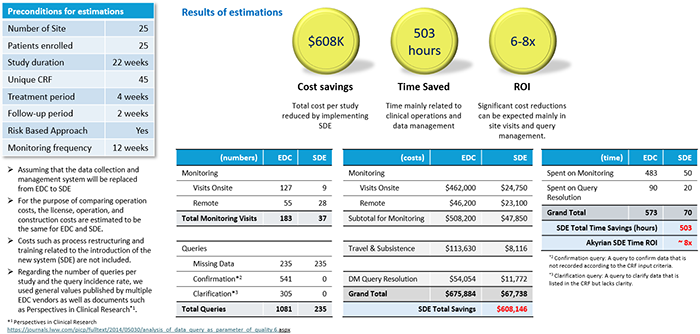
Estimate results for phase II trial
Estimate results for phase III trial
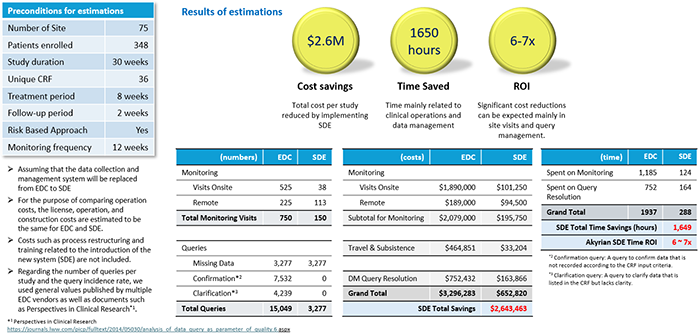
Both Phase II and III trials have shown significant reductions in costs associated with clinical monitoring, particularly on-site visits and associated travel costs. It is also a very interesting result that it is expected to have a significant effect on query response on the data management side. According to this calculation, when comparing phase II and phase III trials, the phase II trial showed a higher ROI, which could be interpreted as having influenced the scale of the trial. It is possible, but the difference is not so large that it is considered to be within the margin of error.
In any case, similar to the Saama's solutions explained last time, the introduction of a new solution that utilizes AI is compared to the improvement rate of 10% or 20% with the introduction of a conventional system. Therefore, I think it is worth paying attention to because it has the potential to bring about dramatic improvement effects.