If you search "AI and clinical trials" on Google right now, you will see that AI is beginning to be used for synthetic control arms, subject recruitment, and site selection, and is beginning to produce some results. Many of them (with a few exceptions, such as Medidata's SCA) use real-world data to train the AI, and it is effective in the design and implementation planning stages of clinical trials.
Recently, when I was talking to my friend living in the United States, he told me that AI models are now able to rank and create lists of appropriate candidate sites for new clinical trial more quickly and accurately than experienced feasibility check experts and site selection specialists at pharmaceutical companies and CROs. He said, "It's faster than a human being can actually do it." This AI model is one example of how highly specialized skills that previously relied on individual knowledge and experience can be replaced by AI if sufficient data is prepared to support them.
As you can see, various solutions that utilize AI are beginning to be offered in clinical trials, but what about the tasks performed during clinical trials, such as site monitoring and clinical data management?
The solution from Saama Technologies (hereinafter Saama) that we will introduce this time combines AI with data from current clinical trials to streamline processes such as site monitoring, clinical data management, and medical monitoring during clinical trials, and speed up the development itself. This approach is yet to be seen anywhere else.
Saama's solution was also used in Operation Warp Speed, which was implemented in the development of a vaccine for COVID-19 infection, and has a track record of contributing to speeding up development. Let's take a look at the data review efficiency improvement results created based on the data published by Saama (see figure below).
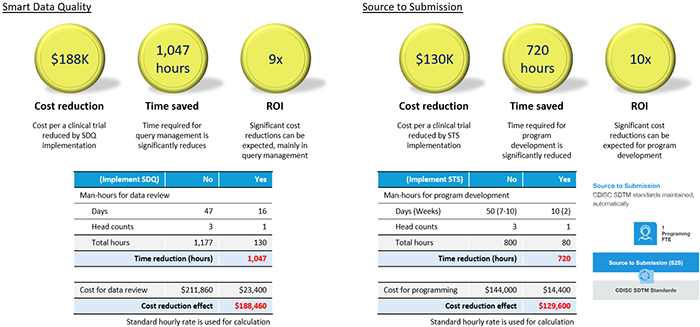
Smart Data Quality (SDQ) is a module used for data review and query management in clinical data management work, and the figure and table on the left show how effective it was for this work. It is noteworthy that an overwhelming 9-fold effect can be obtained in clinical data management work, which takes time even in the clinical trial process.
In addition, the figure and table on the right show that Source to Submission, a module for creating SDTM-formatted datasets used for application, allows programs for creating SDTM datasets to be developed more efficiently than before. This also shows that it is 10 times more effective than before.
In addition, it is important to note that introducing solutions such as SDQ and Source to Submission is more cost-effective than building an AI model from scratch and conducting a PoC. These solutions can be introduced in about two months and have relatively low implementation costs, making them attractive options for pharmaceutical companies. Using AI to improve the efficiency of clinical trials will become increasingly important.
One of the key points in AI use cases is that they produce results that are orders of magnitude greater than the comparisons that have been made in the past when introducing new systems, such as "operational efficiency will double."